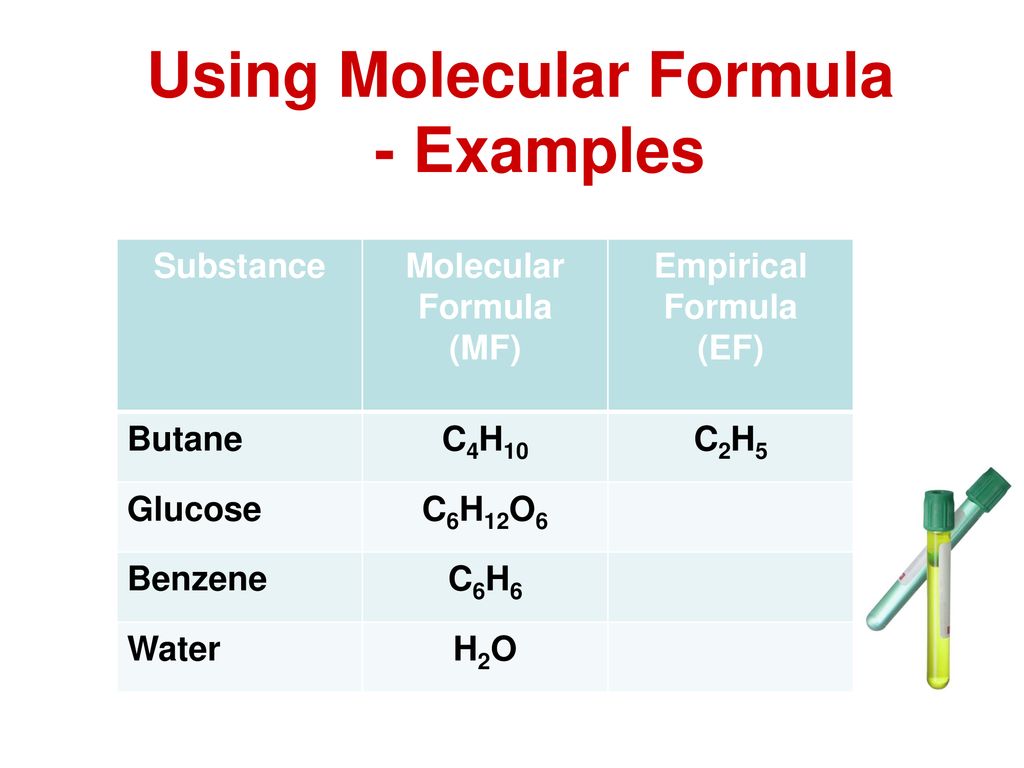
Empirical Formula of Butane
The formula for butane is C4 H10 because hydrocarbons have the formula Cn H2n2. What is the empirical formula for butane eqC_4H_10 eq.
Empirical Formula Of A Compound Ppt Download
For every mole of carbon there are two moles of hydrogen.

. A simple example of this concept is that the empirical formula. The carbon-to-hydrogen ratio equals 23. For every mole of carbon there are two moles of hydrogen.
0 The empirical formula for C4 H10 is C2 H5. What is the empirical formula of butane. The carbon-to-hydrogen ratio equals 23.
C2H3 is the empirical formula for butane C4H6. You burn a sample of. This formula does not.
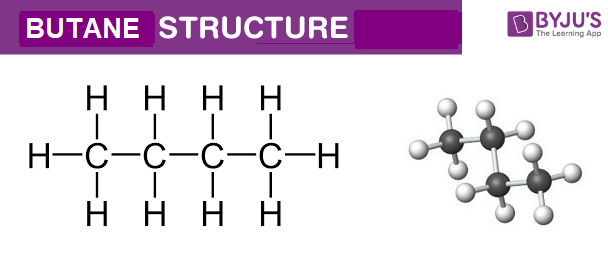
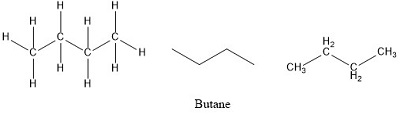
Empirical Formula of Butane Pe_DominicRandall693 September 11 2022 Butane Molecular Geometry Hybridization Molecular Weight Molecular Formula Cas Number. Butane or n-butane is a simple alkane consisting of a chain of four carbon atoms. The molecular formula of butane is C 4 H 10.
In chemistry the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. How are empirical and molecular formulas related. C2H3 is the empirical formula for butane C4H6.
For example butane has an empirical formula of C2H5 lowest whole-number ratio and a. The molecular formula and empirical formula of some substances are the same. Determine the empirical and molecular formulas of each of the following substances.
Moles of H 08247 g H₂O 1mol H₂O 1802g H₂O 2mol H 1mol H₂O 0091 532 mol H. C 2H 5ExplanationThe formula for butane is C 4H 10 because hydrocarbons have the formula C nH 2n2The epirical formula for butane however is C 2H 5. The empirical formula is the simplest ratio of a number of each different atom present in the compound.
For example both types of formula for carbon. Moles of C Moles of H 0036614mol 0091532mol 1 25000 2 50000 2 5. This formula does not.
Hence the molecular formula. The molecular formula of butane is C 4 H 10. This gives the empirical formula of butane - C 2 H 5.
Hint Empirical formula is the simplest formula which provides the lowest whole number ratio of atoms which exist in. Was this answer helpful. What is the empirical formula of.
C 2 H 2 2 CH. The molecular formula for butane is C_4H_10. The epirical formula for butane however is C2 H5.
To determine the molecular formula from the empirical simplest formula you need to compare the empirical formula. This is the actual number of atoms of each element in a molecule of butane. You burn a sample of.
This is the actual number of atoms of each element in a molecule of butane. The empirical formula of butane is CH. FROM COMBUSTION ANALYSIS You can calculate the empirical formula by doing a combustion analysis.
The empirical formula of butane is C₂H₅. The butane formula is given according to the nomenclature rules for alkanes. You can calculate the empirical formula by doing a combustion analysis.
What is the empirical formula of butane. The chemical formula represents the composition of the substance using the elemental symbols and. Molecular Formula n Empirical Formula.
We can simplify the molecular formula C4 H10 which is the formula for butane by dividing the formula. The molecular formula of butane is C 4 H 10. Find the empirical formula of a compound containing 406 carbon 51 hydrogen and 542 oxygen.

Butane Formula Structure What Is Butane Used For Video Lesson Transcript Study Com

Butane Molecular Geometry Hybridization Molecular Weight Molecular Formula Cas Number Bond Pairs Lone Pairs Lewis Structure
Comments
Post a Comment